
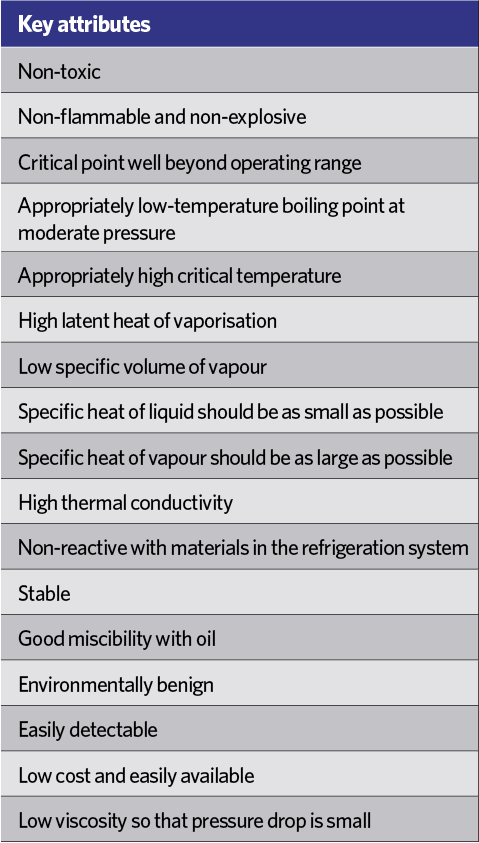
Table 1: Some key attributes for a refrigerant applied in a vapour compression system
The refrigeration machines of the early 19th century used the natural – and ‘green’ – refrigerants of water and air. The early Perkins vapour compression machines of the 1830s used volatile (diethyl) ether, setting the path towards the development of purpose-made chemicals that provided the best compromise for successful applications in refrigeration. There are many attributes (some illustrated in Table 1) that contribute to an effective refrigerant – some of which are tied to particular applications and refrigerants. Many of the early refrigerants, including ammonia, methyl chloride and sulphur dioxide, were flammable, toxic or both.
Working at US company General Motors in the 1920s, Midgley and Kettering developed a non-toxic, non-flammable, stable and efficient chlorofluorocarbon (CFC) – containing carbon, fluorine and chlorine atoms – initially by creating the refrigerant now categorised as R12. This was considered a miracle compound up until the 1970s, when it was established that the use of CFCs was the main cause of ozone-layer depletion. It was also later identified as a contributor to global warming. In 1987, CFCs were banned by the Montreal Protocol, so accelerating the development and application of more ‘ozone-friendly’ refrigerants. R134a, for example, was developed as a replacement for R12 and – as it shares very similar properties – has become commonly used in medium- to large-scale water chillers, as well as in the automobile industry.
However, despite the lower ‘ozone depleting potential’ (ODP) of the hydrochlorofluorocarbons (HCFCs) – containing hydrogen, chlorine, fluorine, and carbon atoms – such as R22 and R123, and HFCs such as R134A and R410A, they have subsequently been identified as significant contributors to ‘global warming’.
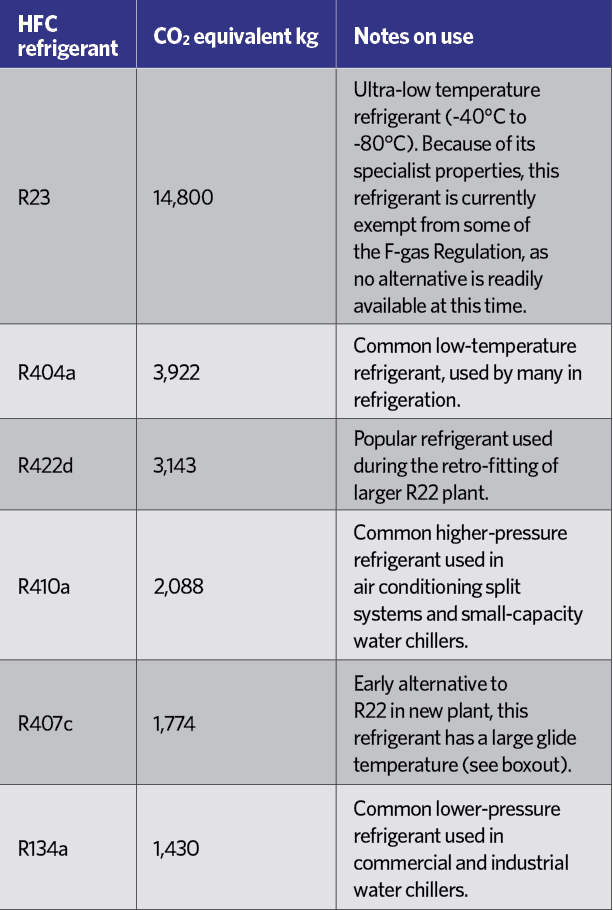
Table 2: Equivalent CO2 value for most commonly used HFCS3
HCFCs have been practically banned since January 20151, and HFCs are rated in terms of their ‘global warming potential’ (GWP) – an equivalent mass of CO2, as shown in Table 2. Although R134a is not considered to have a high GWP, each kilogram is equivalent to 1,430kg of CO2, which in the short-to-medium term is not considered environmentally acceptable, and – in common with other HFCs – it is covered by the EU’s F-gas Regulation.2 This includes a staged reduction in the use of HFCs in the years to 2030. Table 3 indicates the percentage of the HFC baseline that will be available to the EU market each year, moving towards the 2030 target, with specific targeted reductions as shown in Table 4.
The F-gas Regulation phasedown programme is constructed so that – over the next 12 years – end users and manufacturers will be drawn to using refrigerants with a lower GWP, because the production quotas will increase their availability and so make them relatively economical.
CIBSE5 Guide M indicates that water chillers have a life expectancy of between 15 and 25 years, depending on whether they are air- or water-cooled, and on the environment within which they operate. So, for new systems currently being installed, there is a good chance that they will be in service throughout the whole HFC phasedown period. As such, it is timely to evaluate alternative refrigerant systems.
Alternative refrigerants
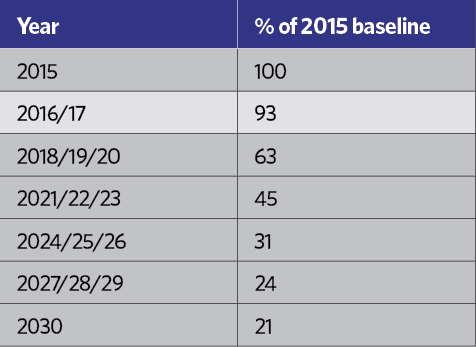
Table 3: Percentage of the HFC baseline that will be available to the EU market each year
There has been a resurgence in the development of modern technologies to make safer use of ‘natural’ refrigerants as an effective alternative to HFCs. These include ammonia R717, CO2 R744 and hydrocarbons – isobutane R600a (often used in domestic fridges and freezers) and propane R290 (used in commercial heat pump and refrigeration applications). These are all successful, but each has operational challenges, such as high pressure and concerns about flammability, explosions and toxicity.
So the quest for a safe, efficient, and – crucially – environmentally friendly refrigerant has continued, leading to the application of hydrofluoroolefins (HFOs), containing hydrogen, fluorine and carbon.
Unlike traditional HFCs and CFCs, which are saturated, HFOs are olefins – so called because they form oily liquids on reaction with chlorine gas – that are unsaturated because of the carbon-to-carbon double bond in their structure. HFOs are relatively stable compounds, but are more reactive than HFCs because of the reactivity of the carbon–carbon bond. This reduces their GWP, with an atmospheric life of 18 days compared with the 13 years of HFC R134a. However, the zero ozone-depleting HFO still mimics much of the basic performance and properties of R134a.
HFO refrigerant as a ‘green’ replacement for HFCs
The principal HFO that has been applied for non-vehicle refrigeration is R1234ze(E), while R1234yf, typically, has been applied in vehicle mobile air conditioning (MAC). R1234ze(E) has operating conditions and costs in line with R134a – as indicated in the pressure-enthalpy diagrams for R134a and R1234ze(E) in Figure 1, and the key attributes in Table 5 – but with ultra-low GWP. Its performance is such that it can replace R134a in new equipment, where its lower volumetric capacity can be addressed in the design of the equipment.
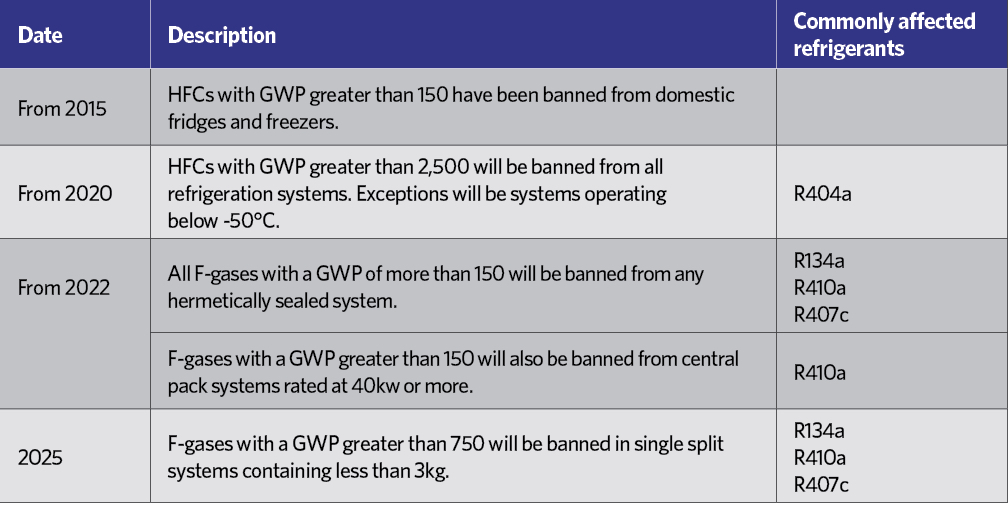
Table 4: Phasedown programme for HFC refrigerants4
A number of concerns have arisen about the safety of HFO refrigerants, including issues such as flammability, potential of hydrogen fluoride formation and formation of trifluoroacetic acid (TFA).
- HFOs have been identified as being flammable under specific non-operational conditions. However, in terms of operational safety, ASHRAE has classified7 R1234ze(E) in refrigerant safety group A2L (lower flammability and lower toxicity), and it is classified as non-flammable for handling and storage below 30°C. The basis of flammability is evaluated by ‘chance of flame occurring’ and ‘effect of flame occurring’ at 60°C. R1234ze(E) needs 250,000 times more energy than propane to ignite and, should a flame occur, it would be low heat (20% of that of propane), with a low burning velocity.
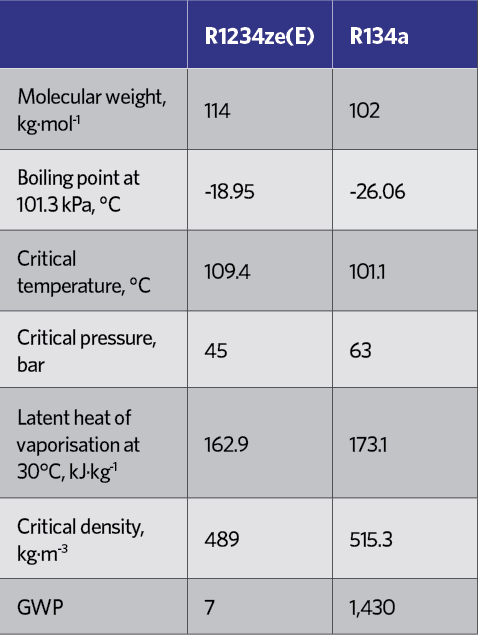
Table 5: Comparison of key attributes between R134a and R1234ze(E)6
- In the presence of high temperatures (as might occur in a fire), all halogenated hydrocarbons – that is, hydrocarbons that include a halogen such as chlorine or fluorine – readily decompose to form hydrogen fluoride. This gas is highly soluble in water, forming acid that can present an extreme safety hazard. Ito8 analysed the production of hydrogen fluoride from an HFO and common HFCs across a range of high temperatures, and the overall results in these early studies indicate that there is little practical difference between the refrigerants.
- TFA is a potentially toxic by-product of the atmospheric degradation of fluorocarbons. It is removed from the atmosphere by wet deposition and is known to accumulate in certain ecosystems. It is estimated that 7-20% of R134a emissions degrade to TFA. Unlike R1234yf (that is principally employed in MAC), which reacts much faster and decomposes completely into TFA, less than 10% of HFO-1234ze(E) is likely to decompose into TFA.9
R1234ze(E) performance in chillers
The volumetric refrigerating capacity of R1234ze(E) is below that of R134a and it has a higher boiling point. So R1234ze(E) cannot be considered as a drop–in replacement of R134a, but, instead, should be considered in new equipment designs.10
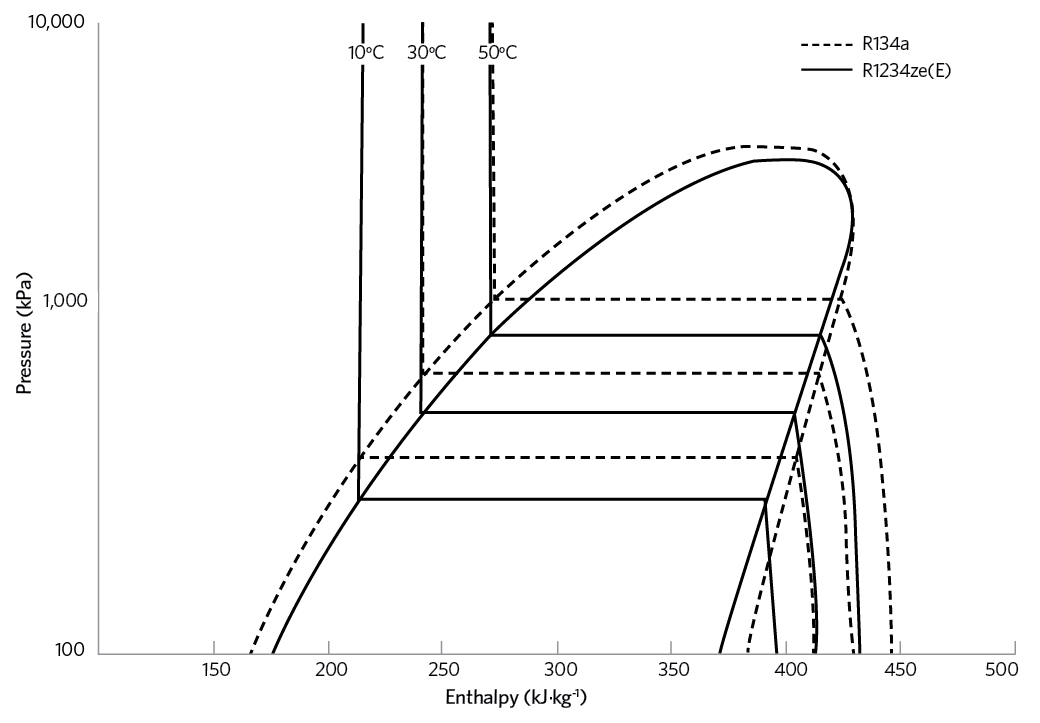
Figure 1: Pressure-enthalpy diagrams for R134a and R1234ze(E)
Field tests of optimised chillers designed specifically to operate on R1234ze(E) have confirmed that the coefficient of performance (COP) is higher than the HFC refrigerant system it is designed to replace. Operational tests have found that R1234ze(E) consumes significantly less energy than R290 (propane) – its closest ultra-low GWP rival – but without the same flammability risk and consequent additional safety considerations. Table 6 shows the comparative performance of three identical capacity and footprint air-cooled chillers. The R1234ze(E) chillers in this comparison have been under development by the manufacturers since the ready availability of the refrigerant in 2012.
Similarly favourable results were reported by Kabeel et al12 in 2016, when they undertook an experimental study into employing R1234ze(E) for a walk-in cold room.
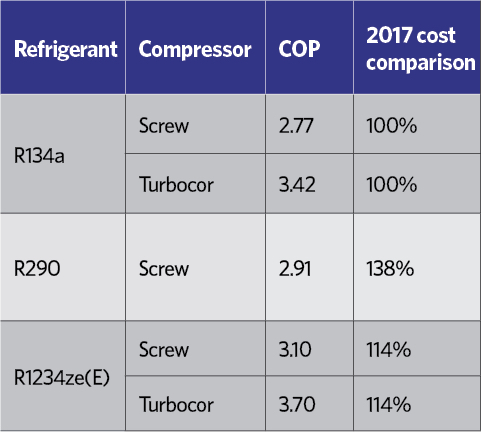
Table 6: Example comparative performances of commercially available air-cooled chillers for 300kW capacity, ambient air 35°C, chilled water 6°C flow, 12°C return11
It is considered that the differential in the costs of the R1234ze(E) compared with that of R134a will disappear over the next 24 months, as production volumes of the HFO refrigerant increase and the F-gas quotas start to affect R134a prices.
HFOs can also be used as a component to produce a lower GWP refrigerant mixture containing HFCs. Several of these are already supported by some compressor manufacturers, but reliability issues have been raised because of high compressor discharge temperatures compared with their HFC predecessors.
This is an exciting time for the application of novel refrigeration solutions for building services engineering. To meet the legislative and environmental demands, there are several developing technologies – both traditional and new. Meanwhile, the application of recently evolved refrigerants, such as R1234ze(E), can meet the demands of both low ODP and low GWP, while maintaining good COPs, and so reducing consequent CO2 emissions.
© Tim Dwyer, 2017.
- Thanks to Robert Young of Cooltherm for his contributions to this article.
